BIOELECTRONICS
Transcutaneous Bilirubinmeter
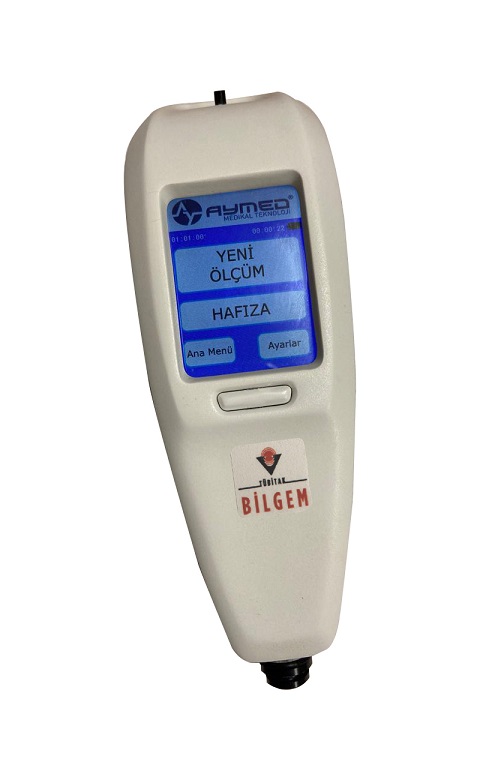
The Bilirubinmeter is a device that can test jaundice in newborn babies with optical measurement through the skin, has a completely unique software, hardware and optical architecture, is suitable for routine use, works fully automatically, can transfer results to patient records wirelessly, and provides rapid intervention without effort and pain to the baby, and is also the first bilirubin measurement device developed with domestic and national facilities in Turkey.
In the traditional method, measuring jaundice in newborns is done by taking a blood sample from the baby. However, for babies requiring continuous monitoring, frequent blood sampling can be cumbersome and painful. To address this issue, a multidisciplinary team was formed at TÜBİTAK BİLGEM UEKAE Bioelectronic Systems Laboratory to develop a bilirubin (jaundice) measurement device that can perform measurements entirely painlessly using an optical method.
Neonatal jaundice is visible when bilirubin, a yellow pigment, accumulates in the subcutaneous region. The severity of neonatal jaundice is proportional to the concentration of bilirubin. As the concentration of the bilirubin substance increases, the color of the skin becomes more yellowish. Reflection spectroscopy can be used to quantify the degree of yellow color of the skin. In order to design the optical unit of the device, the mathematical and physical model of the baby's skin was created. In line with the model, the optical unit and the mechanical design suitable for the optical unit were made and the production of the device was started.
In order to design the optical unit of the device, a mathematical and physical model of the baby skin was created. In line with this model, the optical unit and the mechanical design suitable for the optical unit were made and the device production started. The device is the first medical device developed by TÜBİTAK BİLGEM. The technology transfer agreement for the device was signed with AYMED Medikal Inc. All necessary approvals (Ethics Committee, CE, etc.) for the mass production of the device have been obtained by the company and hospital tests have started. In the near future, the company will start mass production of the device and domestic sales will begin.
The features of the device are as follows:
- Its software, hardware and optical architecture is 100 % original.
- It is suitable for routine use.
- It works completely automatically.
- It can transfer results to patient records wirelessly.
- It can test jaundice in newborn babies by optical measurement over the skin.
- Rapid intervention can be done effortlessly and without causing pain to the baby.